The efficacy of sunscreen products in protecting consumers from the undesirable effects of the sun on the skin (sunburn, photoaging, skin cancer) is well established.
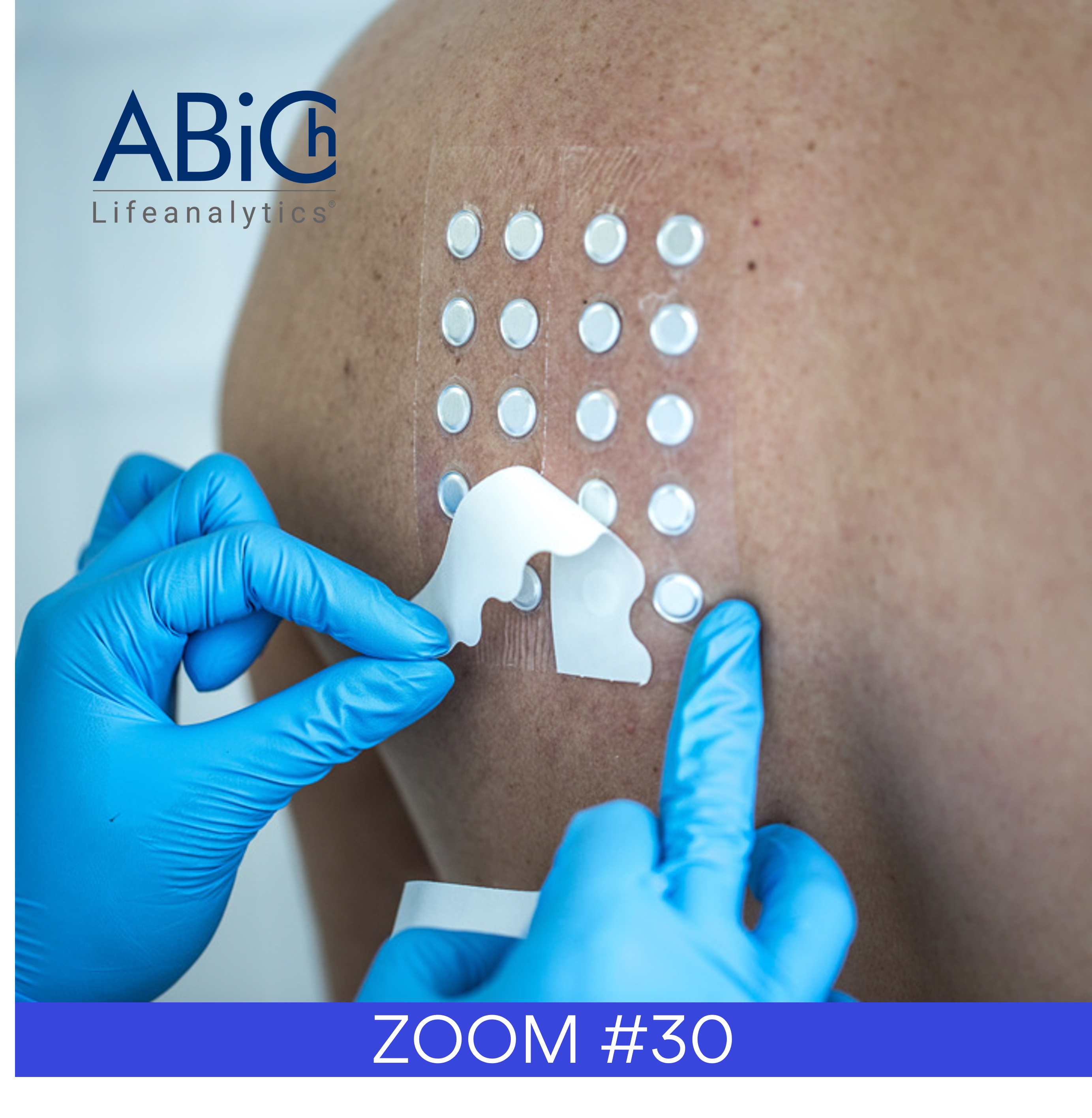
In terms of evaluation, sunscreens are unlike other cosmetic products as they must conform to standardised and internationally-recognised methods for efficacy testing. At the end of 2019, the ISO/TC 217 Cosmetics committee published a technical revision of the ISO 24444 – In vivo determination of the sun protection factor (SPF). The main changes are as follows:
– Revision of the definition of the minimal erythema response (MED) criteria;
– Modification of lighting conditions for visual determination of DEM;
– Choice of eligible test subjects now based solely on individual typology angle (ITA°);
– ITA° used to define the range of unprotected MED doses;
– Validation of three new reference standard sunscreens for products with SPF equal to 25 or higher (P5, P6 and P8);
– Description of new test methods to determine the uniformity of the beam of solar simulators;
– Description of sunscreen application procedures in greater detail;
– Addition of an informative annex, with photographic examples of erythema responses and guidelines for grading;
– Modification of the reporting tables and requirements to provide more complete information on results of testing;
– Bibliography update;
These modifications aim to reduce the remaining variability between test laboratories, in order to obtain similar FPS regardless of the laboratory that carried out the evaluation.
Revisions to standards ISO 24442 – In vivo determination of UVA protection, ISO 16217 – Water immersion procedure for determining water resistance as well as ISO / FDIS 18861 – Percentage of resistance to water are also expected this year.
As active participants in international technical review committees, solar experts from Eurofins Cosmetics & Personal Care laboratories participate in validation cycles of various standards relating to solar tests (whether in-vivo or in-vitro) and facilitate their implementation in Eurofins sites. Consistent inter- and intra-laboratory harmonisation guarantees homogeneous test methods for all solar products and optimal quality across all our sites. This optimises the performance of multicenter solar studies.
Our regulatory expertise, coupled with our years of experience in toxicology, physico-chemical analyses, ecotoxicity, biodegradability, and OTC product support make Eurofins Cosmetics & Personal Care THE solar center of excellence!
Read the complete article: FOCUS#2 Solar Testing – Skinobs
cosmetics@eurofins.com









 Follow us on Linkedin!
Follow us on Linkedin!
You must be logged in to post a comment.