Oct 28, 2015 By: S. Miksa, D. Lutz and C. Guy, HelioScreen, Creil, France –
Editor’s note: Proposed here is an in vitro method to test sunscreens for photostability. Part I sets up the protocol, Part II details the test method, calculations and results, while Part III outlines a new photostability label claim concept based on the results.
Detailed Test Method
Using these described steps, 107 products were assessed as follows.
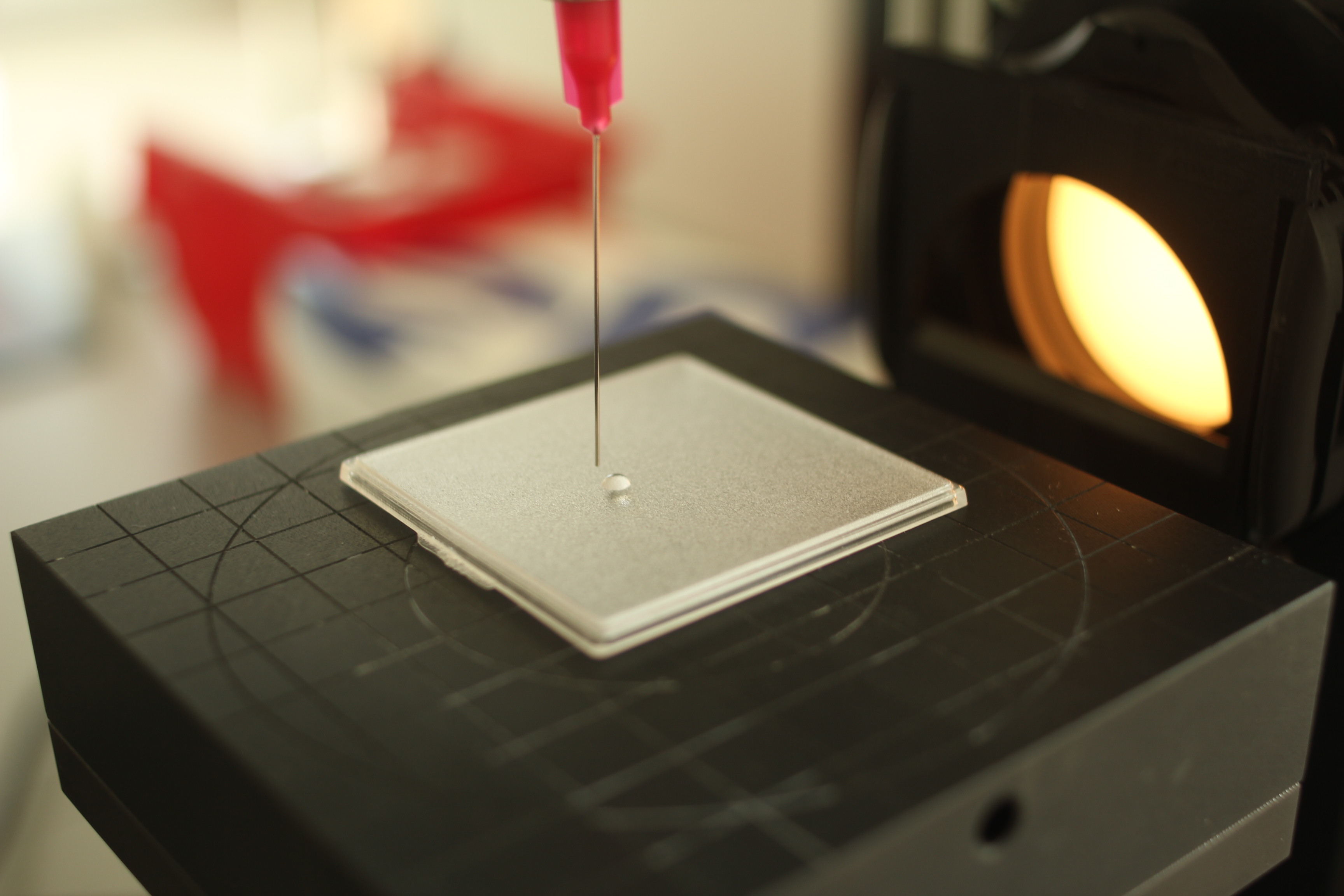
Product application: Each product was spread using an automated syringe across a substrate/plate surface at a rate of 1.3 mg/cm². Per the ISO 24443:2012 Standard, PMMA platesa having one reproducibly rough side to replicate topography parameters were used to ensure fairly consistent film-forming.21 The substrates were positioned in a horizontal plane so as to avoid uneven flow of the sunscreen, which could give varying results.
Immediately after weighing, the sunscreen was spread over the whole substrate surface using an automated robotb to ensure total reproducibility; previous work19 has shown manual spreading to cause variations in test films, challenging the reliability of results. Note that alternatives to robotic spreading may be used but should produce results similar to, comparable with and correlated to those achieved using robotic spreading. A similar number of products also must be used, in this case 107, to provide accurate and relevant results.
Temperature control: During spreading, the temperature of the substrate/product interface was controlled by a devicec designed to maintain 25°C ± 0.5°C.20 After the product was spread, the sample was allowed to dry and settle for 30 min in the dark for self-leveling, all while maintaining 25°C. As noted, strict control of the product/substrate interface temperature is compulsory for thermodynamic reasons, as fluctuations could challenge the relevance of this method. Control over the room temperature may also be useful but is not crucial.
UV transmittance: Before transmission tests, the transmittance analyzerd was calibrated both by internal specified standards and the linearity/additivity by calibrated reference standard PMMA platese, to which UV filters were incorporated.22 After samples were dry, their UV transmittance was measured. Blank transmittance also was obtained by covering a PMMA plate with a film of white petrolatum.
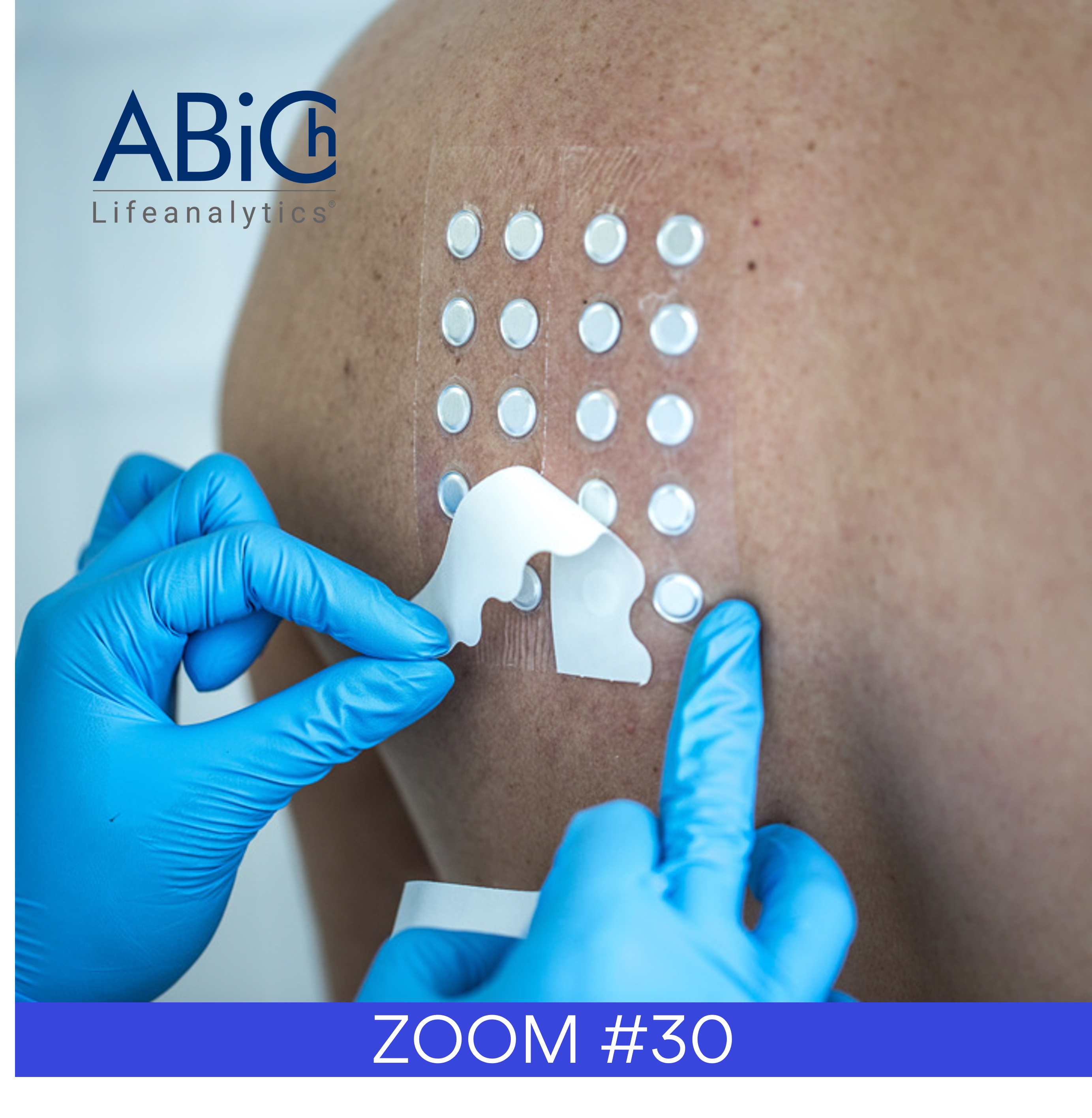
Three different plates were used per product, and nine UV transmission spectra were recorded at every nanometer from 290-400 nm for each plate at different application locations. For the present method, a minimum of three replicates at different placements on the plate were measured, representing a total 20 cm² of surface area.
Irradiation intensity: Prior to irradiationf, the intensity of the UV exposure source must be checked by means of a spectroradiometer or radiometerg sensitive to UVB erythemal effectiveness (see Erythemal Effectiveness sidebar). Of the total 290-400 nm UV range, the radiometric proportion of UVA II (320-400 nm) should be higher than 20%, and the proportion of UVA I (340-400 nm) should be higher than 60%, with respect to limits of the percentage of relative cumulative erythemal effectiveness (RCEE) (see Table 1).
UV irradiation: Samples were then exposed to the full spectrum of UV radiation according to doses of 0, 2, 4, 6, 8, 10, 12, 14, 16, 18 and 20 MEDs to determine product photodegradation categories; note that only 0, 4 and 8 MEDs are used in the final method, as explained in the next section of this paper.
While the erythemal power of UV radiation can be measured in terms of effective irradiance, as defined by UV A+B content, it is also related to the sensitivity of human skin to sunburn, expressed as Minimal Erythemal Doses per hour (MEDs/hr). Here, the well-accepted definition for an MED unit is used; i.e., the dose of UV exposure capable of causing minimum skin redness (erythema) in average type II skin: 200 J/m²-eff (erythema-effective Joules (J)/m²).
During UV irradiationf, the test procedure must take only the eventual photodegradation of the sunscreen into consideration. External parameters may not be imposed, according to specification published;18 for example, the sample must not be cooled by air flow, and the temperature during exposure must remain at 25°C ± 2°C.
Calculations
Before and following each UV irradiation step, transmittance measurements were taken using the same applianced at the same location for each plate. To determine the ability of each product to absorb or scatter UV light, monochromatic attenuation factors were evaluated at each wavelength between 290 nm and 400 nm; the monochromatic attenuation factor is calculated as the inverse of the value of transmission: 1/T(λ).
To express the percentage of residual efficiency for UV A+B parts, referred to as UVA+B %RE, Equation 1 was used, where n represents the number of plates.
Here, T(λ)0 is the transmission before irradiation, and T(λ)t is the transmission after irradiation. The same equation is used for any UV irradiation dose.
From a statistical point of view, the UVA+B %RE of the product is the arithmetic mean of individual plate UVA+B %REn values, obtained from the total number of plates used, n, and expressed to one decimal point according to Equation 2.
The standard deviation (SD), s, is obtained by Equation 3.
The final UVA+B %REf retained for photostability labeling is determined as follows:
1. Calculate the mean UVA+B %RE and the standard deviation, s, from the UVA+B %REn values.
2. Calculate the standard error (SE), which equals s/√n; where n equals the number of plates that provided valid test results.
3. Calculate the t value from Student’s t distribution table corresponding to the upper 5% point, with n-1 degrees of freedom.
4. Obtain the final UVA+B %REf, which equals the largest whole number less than UVA+B %RE – (t*SE).
Photodegradation Results
In the first step of this process, the photostability behaviors of the products were assessed and three common evolutions of residual efficacy were observed, depending on the photo-behavior of the product (see Figure 1): a) no photodegradation, b) linear photodegradation, or c) polynomial photodegradation.
Furthermore, different photodegradation behaviors were observed when viewing only UVB (290 nm to 320 nm) (see Figure 2); only UVA (320 nm to 400 nm) (see Figure 3); or both UV A+B (290 nm to 400 nm) (see Figure 4). These results confirm the need for product developers to consider the whole UV A+B spectrum absorbance for photostability calculations and consumer safety. Figure 5 attempts to summarize the photostability results for all 107 products.







 Follow us on Linkedin!
Follow us on Linkedin!